by Philippe Tacon, Global Aquaculture
Manager, Phileo
First published in International Aquafeed,March-April 2015
A survey made at the end of an aqua
industry forum meeting in Vietnam last year has shown that for 63 percent of
the participants, the most limiting challenge for developing aquaculture was
health and disease management. Indeed, in recent years, we have seen numerous
diseases appearing and impacting aquaculture production, such as WSSV and EMS
in shrimp, or Infectious Salmon Anemia (ISA) in salmonids. Working around the
classic Host-Pathogen-Environment triad, new technologies and management
techniques have been developed to better control diseases in aquatic animals:
vaccination, which has led to the decrease of antibiotic use in salmonids;
biosecurity procedures in hatcheries and in farms; biofloc technology. All of
these technologies have proven successful. Their further development and
expanded use will certainly improve the way aquatic animals are farmed.
Another strategy is to increase the health
of the animal through feeding, and this magazine might be a good place to
discuss it. Well balanced diets can certainly improve the health status of a
fish or a shrimp, but in some challenging conditions, like a pathogen
infection, the use of immune stimulants can be required to enhance the response
of the immune system.
When studying immune stimulation, it is important
to understand that the immune system of aquatic animals differs not only
between theirs and the mammalian one but also between teleost and crustacean.
Fish are the first group in which a specific immune system appears in the
evolutionary tree. The fish immune system therefore has a greatly inferior
performance to that of mammals (see Tort et al 2003). It is less specific, less
sensitive and has only oneclass of antibodies (IgM).
Fish being poikilothermic animals, it is
highly dependent on temperature, low temperature slowing down the immune
response up to 10 to 12 weeks. Fish rely by then more on their non-specific
immune system (also called innate immunity) to fight against pathogens. The
innate immune system recognises non-self molecules that could be of foreign
origin - also called pathogen associated molecular patterns (PAMP) - and
molecular patterns exposed though damage to the host. These patterns are
recognised by germline-encoded pattern recognition receptors (PRR) or pattern
recognition proteins (PRP). These molecular patterns can be for example
peptidoglycans and lipopolysaccharides from bacteria cell walls, fungal b1,
3-glucan, viral double-stranded RNA and bacterial DNA (see Magnadottir 2006 for
an overview of fish innate immunity).
Fish innate immunity starts with first barrier defences such as mucus; it traps pathogens and includes lysozymes, antibacterial peptides which can eliminate pathogens. Neutrophils and macrophages are key cells of the innate immune complex as they can phagocytose pathogens (a mechanism which is not temperature dependent) and release Reactive Oxygen species, which are toxic to pathogens. Completing this cellular response, the humoral response implicates the synthesis and release of antimicrobial components.
In shrimp, where the picture is even
simpler as they rely only on innate immunity, we find the same type of
mechanisms in place as in fish with phagocytosis performed by granulocytes (a
specific form of the blood hemocyte cells) and humoral response. However the
most effective mechanism of invertebrates (as arthropods) is cellular melanotic
encapsulation. This requires the combination of circulating hemocytes and
several associated proteins of the prophenoloxidase (proPO) activating system.
Recognition of PAMPs such as LPS and β-1, 3 glucans by PRPs is an essential
step for the activation of the proPO cascade (Amparyup et al 2013).
Stimulation of the innate immune system,
which would enhance the speed and the effect of the immune response, is
therefore possible by mimicking the effect of PAMP on PRR and PRP. In that
regard, beta glucans have been studied for a long time in aquaculture and seem
‘the ideal’ immune stimulant in aquaculture (see Meena et al 2013 and Ringo et
al 2012) as they can specifically activate macrophages in fish and the proPO
cascade in shrimp.
Parietal fractions, such as Safmannan® are extracted from a selected Saccharomyces cerevisiae strain respecting strict EU manufacturing control standards. They contain beta glucans, mannan oligosaccharides that are all activators of the immune system (Song et al 2014).
Earlier internal trials have shown that
yeast cell walls and parietal fractions have different effects in mycotoxin
binding and immunity in aquatic animals. Indeed several trials done at the
Hellenic Center for Marine Research in Greece have shown that yeast fraction
products with similar manna/glucan ratios from Phileo, Lesaffre Animal Care
Business Unit, have very different effects in the stimulation of immune
parameters and in survival following challenge in Vibrio anguillarum.
It looks like not only the mannan and
glucan content is of importance, but the strain and the drying processes are
also key parameters to ensure a good effect in aquatic animals. Another concept
that came out of these trials was that there is a threshold of yeast material
to be ingested before it starts to kick in and improve the immune system.
Product origin, quality, dosages and duration of treatment are all clearly
linked.
A trial has been undertaken to further study
a dose response of Safmannan® in a marine species. The objective was twofold:
investigate the influence of parietal fractions in diets with a reduced amount
of fishmeal, and determine the dosage needed for an optimum immune response (Yu
et al 2014).
Six diets were designed (see table 1): a
high fishmeal diet with 38.5 percent fish meal inclusion and no soybean meal
(HFM) and 5 diets with 25 percent fishmeal and 20 percent soybean meal. These
diets were supplemented with 0 (SBM), 250, 500, 1000 and 2000 g/T of
Safmannan®. Juvenile Japanese seabass (18 g) were selected and distributed into
280 L tanks after 24 h starvation with 30 fish per tank, and six tanks per
treatment. The water temperature was maintained. Fish were fed to apparent
satiation twice daily at 08:00 and 15:00 for 72 days.
At the end of the treatment period fish
were anesthetsed, weighed and viscera and blood were sampled. Intestine samples
from the FM, Y0, Y4 and Y5 groups were removed from 2 fish in each replicate
tank at the end of trial (12 fish per treatment) and processed for histology
analysis (H & E staining). Morphological parameters associated with
SBM-induced enteritis of anterior and distal intestines, including the height
of mucosal folds (HMF), width of mucosal folds, lamina propria and connective
tissue were quantified.
After all samples were taken, 40 fish of each treatment (6–7 fish per tank) were divided into 2 groups and transferred into a still water system with temperature at 26 ± 1 °C. The fish were fed as before and recovered from weighing and sampling stress by a 2-week acclimation. Then they were challenged by intramuscular injection with Aeromonas veronii (CGMCC No. 4274) at 8 × 104 cells/100 g body weight. Ten fish from each tank were sampled for plasma immune parameters two days after challenge and the others (20 fish per treatment) were recorded for 7-day cumulative survival rate without any food.
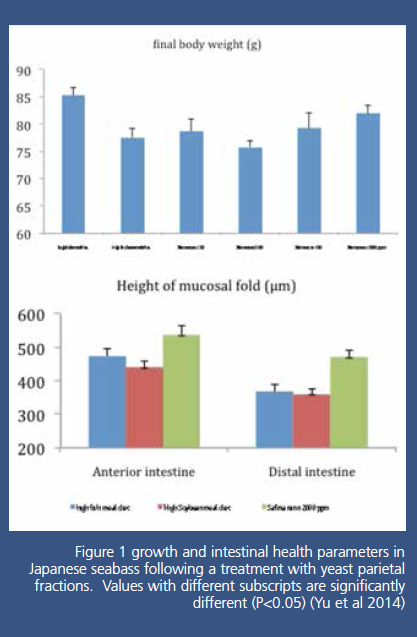
This study showed a lower growth of SBM
diets as expected compared to HFM diets, but an even lower growth with the
500g/T treatment, and a much better growth at 2000 g/T (Fig1). These results
can be correlated to a wider width of mucosal folds in anterior and distal
intestinal in SBM diets compared to HFM diets suggesting a negative effect of
these diet on intestinal health, and also to a higher height of mucosal folds
in the 2000 g/T group (Fig1). This suggests that Safmannan® at 2000 g/T was
able to compensate the negative effect of soybean meal and increase gut health
leading to a better growth.
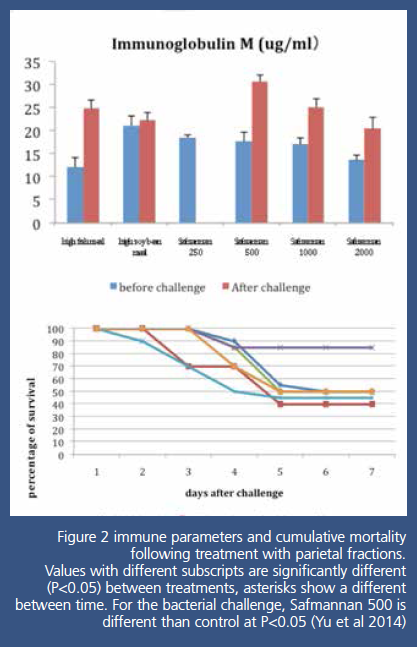
The study also shows that IgM levels were
significantly elevated after the bacterial challenge in the diet containing
parietal fractions at 500g/T (Fig2) indicating a strong immune stimulation. The
levels decrease as the yeast parietal fraction concentration is increased showing
a potential fatigue of the immune system. This is confirmed by the survival of
the fish after the challenge. The optimum dosage was 500g/T of Safmannan®,
whereas higher dosage did not improve survival. Remarkably, we can see this
optimum dosage for immune stimulation was also the one giving the lowest
growth, confirming hypothesis that the strong stimulation of the immune system
is at the expense of the growth potential of the fish.
This study highlights the duality of role
of parietal fractions in fish depending on the dosage and feed composition:
they can be used either as gut health enhancer (high dosage) or immune enhancer
(low dosage).
Formulators and farmers can benefit from
using this efficient and sustainable solution against pathogens but they need
to choose quality products and work with proper (and proven) dosages and
administration durations.
Read the magazine HERE.
The Aquaculturists
This blog is maintained by The Aquaculturists staff and is supported by the
magazine International Aquafeed which is published by Perendale Publishers Ltd
For additional daily news from aquaculture around the world: aquaculture-news


A method of increasing immunostimulation in animals by administering to the animal a glucan product comprising a branched β-(1,3) glucan with β-(1,3)-linked side chains being attached by a β-(1,6)-linkage and being essentially free of side chains containing more than four β-(1,6)-bound glucose units.
ReplyDelete